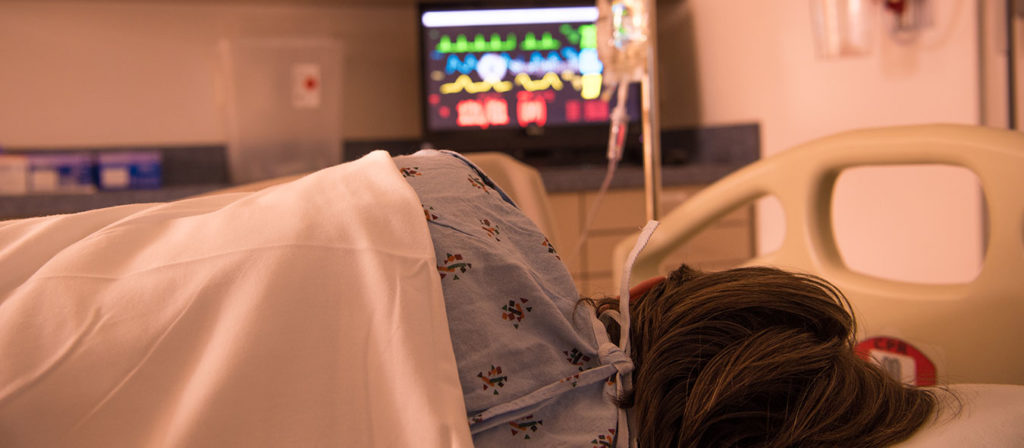
Now that Daylight Saving Time has ended, cyclists are attaching lights to their helmets, and dog walkers are storing flashlights next to their leashes. But one place that won’t get darker with the time change is the hospital. New research out of West Virginia University illuminates how the round-the-clock brightness of hospital rooms may stymie some patients’ recovery.
Randy Nelson, who chairs the Department of Neuroscience in the WVU School of Medicine, and Courtney DeVries, the John T. and June R. Chambers Chair of Oncology Research at WVU, are studying how light at night may worsen outcomes in cardiac patients. Both researchers are members of the WVU Rockefeller Neuroscience Institute. The National Institutes of Health has awarded them nearly $1.2 million for their three-year investigation.
“This research actually grew out of one of our student’s hospital stays,” says Nelson, who also directs basic science research at the WVU Rockefeller Neuroscience Institute. “He said, ‘The lights are on all night. I can’t sleep.’ So we asked the question, ‘What does constant light exposure do to folks in critical-care areas, like cardiac intensive care or surgical step-down units, where people are already immunocompromised?’”
Cardiac arrest doesn’t just hurt the heart. It also hurts the brain. One way this happens is that cardiac arrest and the subsequent oxygen deprivation inflame the nervous system and overstimulates brain cells so much that they can die.
Nelson and DeVries suspect that spending nights in hospital rooms that never get truly dark can amplify these responses. Earlier work they conducted with preclinical models suggests that a light no brighter than a child’s nightlight may be enough to trigger inflammation in the brain, “overload” brain cells and make cardiac arrest more lethal.
Building on their previous findings, the researchers will now explore the physiological mechanisms that link light at night to neurological damage—and a greater mortality rate—after cardiac arrest. They also want to determine whether certain wavelengths of light are more detrimental than others.
To do this, they will expose animal models of cardiac patients to different amounts and wavelengths of light at night. They will then measure the models’ brain inflammation, determine the extent of brain-cell damage and evaluate how nimbly the heart can beat faster or slower to meet the body’s changing needs. Next, they will pinpoint which lighting environments correlate to the best health outcomes.
“The circadian system is affected by short-wavelength light, which is blue, like from your phone or tablet. Fluorescent light is also quite bluish. But if we can use longer-wavelength lights—sunset colors—that doesn’t affect the circadian clock,” says Nelson. Perhaps replacing the fluorescent bulbs in hospital rooms with sunset-toned bulbs would enable the hospital staff to administer drugs, operate equipment and monitor patients at night without disrupting their circadian clocks and hampering their recovery from cardiac arrest.
Light at night seems to be associated with a range of conditions, including obesity and diabetes. But “unlike a lot of things we’ve studied so far with dim light at night,” says DeVries, “the problem after cardiac arrest is that, if the neurons die, they’re dead. It’s not reversible. We’re interested in preventing that.”
Originally from Cassie Thomas for WVU Today.